First gene therapy treatment for a hereditary form of deafness in the USA
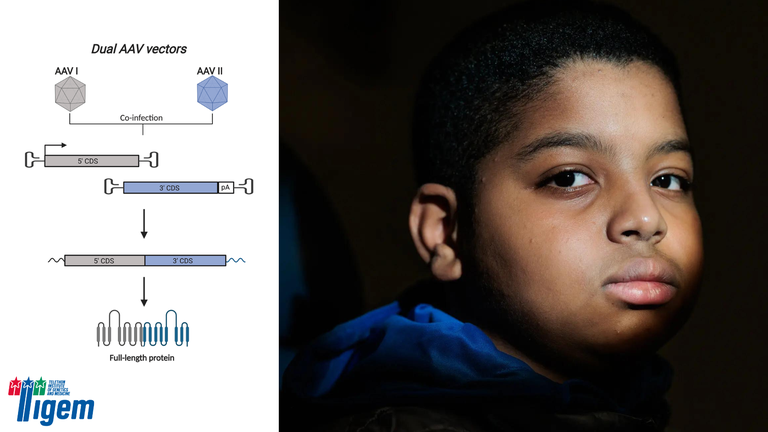
The Children’s Hospital of Philadelphia successfully administered the first gene therapy for hereditary deafness in the U.S. in an 11-year-old boy. This groundbreaking treatment targeted a form of deafness caused by otoferlin gene variants, resulting in significant hearing improvement. Key to this achievement was the dual vector technology developed by Alberto Auricchio, head of the Molecular Therapies program at the Telethon Institute of Genetics and Medicine (TIGEM) and professor at the University of Naples, Italy.
Auricchio’s research overcame the capacity limitations of viral vectors, a crucial hurdle in gene therapy, especially for diseases associated with large genes. His team’s work, supported by the Telethon Foundation Italy and the European Research Council, led to the development of two platforms enabling the division of genetic material into multiple viral vehicles. This technology, licensed to Akouos Inc., a subsidiary of Eli Lilly, is instrumental in expanding gene therapy’s potential to a broader range of genetic diseases.
The success in Philadelphia highlights the impact of Auricchio’s contributions and the promising future of gene therapy for numerous conditions previously considered unfeasible for such treatments.
AAVantgarde, a company founded by Alberto Auricchio, is currently validating its platforms in two lead programs in Ophthalmology with a high unmet need, including one on retinitis pigmentosa associated with Usher Syndrome Type 1B (USH1B), in which the dual hybrid platform is being tested. A clinical trial for this program is set to start soon.