Nacuity announces positive clinical results: oral treatment NPI-001 reduces photoreceptor loss by over 50% in Usher syndrome patients
Thursday 11th September. A major step forward for the Usher Syndrome community. A day that may change the future for thousands of children and families around the world.
The U.S.-based biotechnology company Nacuity Pharmaceuticals has officially announced the results of the SLO-RP Phase 1/2 clinical trial, showing that its oral treatment NPI-001 can reduce photoreceptor loss by more than 50% in patients with retinitis pigmentosa associated with Usher syndrome.
The study was conducted in Australia with 49 participants over a two-year period and provides strong clinical validation of the neuroprotective potential of this first-in-class therapy.
An Oral Neuroprotective Therapy
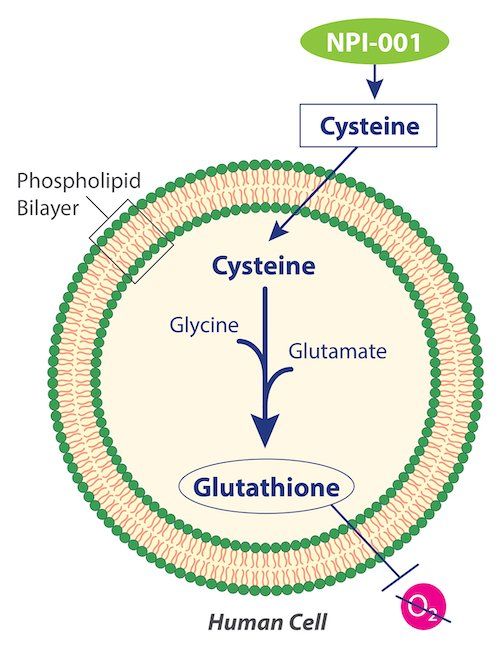
NP-1 is an oral formulation based on a powerful antioxidant molecule with neuroprotective properties, designed to preserve the function of retinal photoreceptor cells and slow down their degeneration over time. Preclinical studies in animal models had already demonstrated strong preservation of retinal structure and function, and now, the early results in patients confirm safety and promising signs of clinical efficacy.
This is the first treatment capable of protecting the retina in people with Usher syndrome.
And that means we gain time.
About the SLO-RP Trial
The SLO-RP clinical trial is a multicenter, randomized, double-masked, placebo-controlled Phase 1/2 study currently underway at several international sites. The protocol was recently amended to allow continued access to treatment for participating patients, even as the study proceeds.
NP-1 has been granted Orphan Drug Designation, providing a 7-year market exclusivity window in the U.S. upon potential approval, and underscoring the need for such innovation in the rare disease space.
🔗 Learn more about NP-1 and the science behind it (Nacuity website)
Key results:
-
Over 50% reduction in photoreceptor loss over 2 years (measured by EZ Area using OCT)
-
Favorable trend (~30%) toward slower loss of visual function (measured by MAIA microperimetry)
-
Demonstrated safety, with no persistent treatment-related adverse effects
-
High adherence to treatment (>80%)
-
These results informed the design of a confirmatory trial planned for 2026
Time Becomes Our Ally
This is the first time a small-molecule treatment has shown an objective therapeutic effect in RP. Being gene-agnostic, it holds potential to benefit a wide range of patients, regardless of the specific mutation involved.
For affected individuals, pediatric Community and families this is nothing short of a narrative shift. Until now, our hopes were pinned on future curative or gene-based therapies. But with NP-1, we are finally talking about preserving current vision, about buying time, about extending independence and quality of life while we continue pushing research forward.
This extra time could mean everything: more children preserving more vision, for longer.